Introduction: Individuals with human immunodeficiency virus (HIV) face a substantially greater likelihood of developing cancers compared to the general population. The prevalence of cancer diagnosis among HIV-positive individuals ranges from 25% to 40%. Non-Hodgkin lymphoma (NHL) stands out as the most prevalent cancer type associated with people living with HIV/AIDS (PLWHA). NHL primarily manifests in two forms in PLWHA: Burkitt lymphoma, which poses an 18-fold higher risk, and diffuse large B cell lymphoma (DLBCL), accounting for 75% of cases. The advent of chimeric antigen receptor (CAR) T cell therapies, notably CD19-targeting CAR T cells, has emerged as a groundbreaking treatment for B cell NHL. Unfortunately, PLWHA has been excluded from participating in all of these clinical trials for years due to safety concerns and the challenges associated with manufacturing CAR T cell products from HIV-positive donors. Recent researches explored the feasibility of developing CD19-targeting CAR T cell products utilizing cells from HIV-positive donors with lymphoma. Although these studies have provided promising evidence that alleviates concerns regarding the safety profile and limited effectiveness of CAR T cell therapy in this particular population, they do not address the underlying HIV condition. We previously generated CAR T cells targeting either lymphoma (CD19-CAR) or HIVgp120 (N6-CAR) that show efficacy against their respective diseases in clinical and preclinical testing, respectively. We hypothesized that a dual construct capable of targeting both antigens could simultaneously target lymphoma cells and HIV-infected cells in a same individual. We report our experience developing a bi-specific N6-huCD19 CAR T cell platform that can target both antigens in a single therapeutic product.
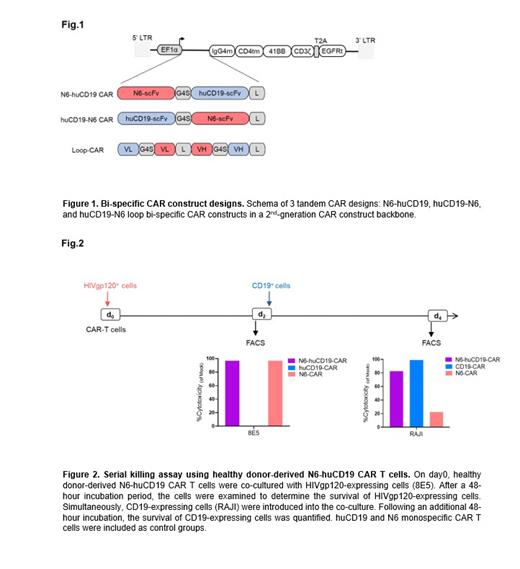
Methods: We engineered 3 bi-specific CAR candidate designs including 2 tandem and 1 loop constructs. These constructs were developed by integrating a humanized (hu) CD19 single-chain variable fragment (scFv) and an N6 scFv from an anti-HIV broadly neutralizing antibody (bNAb) into a backbone of a 2 nd-generation CAR construct. The bi-specific tandem CAR constructs consisted of N6 and huCD19 scFvs fused with a G4S linker in either huCD19-N6 or N6-huCD19 orientation. The loop-CAR was generated by fusing huCD19(VL):N6(VH):N6(VL):huCD19(VH) with a whitlow linker. All constructs included the CD4 transmembrane domain, a double-mutated IgG4 Fc spacer, 4-1BB co-stimulatory and CD3-zetta signaling domains, and EGFRt separated by a T2A ribosomal skip sequence (Fig.1). We tested the 3 bi-specific constructs in healthy donor-derived T cells using cytotoxicity co-culture assay against either Raji (CD19+) or 8E5 (gp120+) target cells. We generated N6-huCD19 CAR T cells from HIV-positive patients and confirmed the functionality of these CAR T cells against tumor cells.
Results: The functional assessment of all 3 bi-specific CAR constructs revealed their efficacy against CD19 and HIVgp120 antigens. However, the N6-huCD19 tandem CAR T cells demonstrated comparable and better efficacy against both antigens. Notably, the N6-huCD19 tandem CAR T cells exhibited the capability to sequentially target either a single antigen or alternate between the 2 antigens (Fig.2). We successfully generated N6-huCD19 tandem CAR T cells using HIV-positive donors, signifying the practicality of this approach within the context of PLWHA. Remarkably these HIV donor-derived N6-huCD19 CAR T cells effectively killed tumor cells expressing CD19 or HIVgp120 antigens, thus confirming their dual functionality against these 2 antigens.
Conclusion: Here we demonstrate the development of the novel N6-huCD19 bi-specific CAR T cells with functionality against both CD19 and HIVgp120 antigens. We also showed the feasibility of generating these CAR T cells from HIV-donors. This strategy could potentially provide life-saving approach tailored for treatment of HIV-patients who develop CD19+ lymphomas.
Disclosures
Nakamura:Jazz Pharmaceuticals: Consultancy, Other: research collaboration; NCCN: Other: guideline panel for HCT; Leukemia & Lymphoma Society: Other: grant reviewer; International Consortium: Other: consortium chair; Mt. Sinai: Other: Acute GVHD; Blue Bird: Consultancy; Napajen: Consultancy; Miyarisan: Research Funding; Omeros: Consultancy; NCTN Lymphoma Steering Committee: Membership on an entity's Board of Directors or advisory committees; BMT CTN Steering Committee: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy. Baird:Kite Pharma-Gilead: Research Funding, Speakers Bureau; Genentech-Roche: Research Funding; Regeneron Pharmaceuticals: Research Funding; Cellular Biomedicine Group: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal